Sanders-Brown publication makes cover of recent Journal of Neuroscience issue
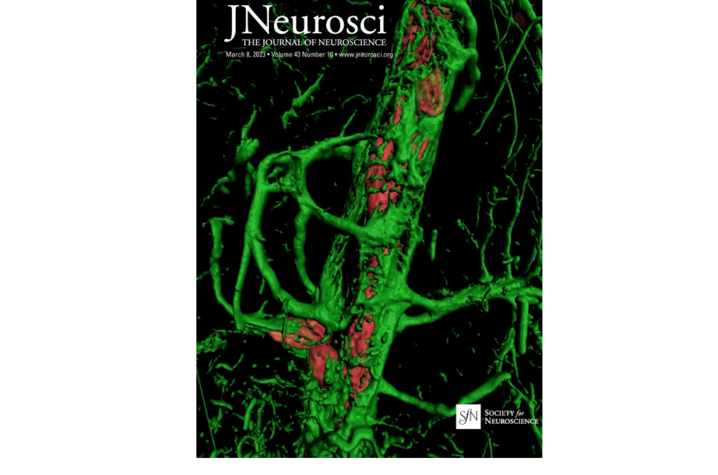
The first published work coming from a multi-million-dollar grant received last year by a team from the University of Kentucky recently made the cover of the Journal of Neuroscience.
The image captured by Pradoldej “Bob” Sompol, Ph.D., shows astrocytes surrounding a blood vessel in the brain of a mouse model from their study. Landing the cover image on a high-impact journal is a first for Sompol and the rest of the team.
Astrocytes are a principal focus of a $20.5 million grant awarded to researchers at UK’s Sanders-Brown Center on Aging.
The large project is named "Strategies for Targeting Astrocyte Reactivity in Alzheimer’s Disease and Related Dementias (STAR-ADRD)." The acronym serves a double meaning as astrocytes — the focus of their project — are star-shaped cells.
Alzheimer’s disease and other forms of dementia are diseases caused by various pathologies. With this grant the research team plans to study the role of astrocytes in these disorders.
“Astrocytes are, in some ways, a forgotten cell in neurodegenerative diseases,” said P01 leader Chris Norris, Ph.D., professor in the UK College of Medicine’s Department of Pharmacology and Nutritional Sciences, and an associate director at SBCoA. “For many years they have been thought of as support cells for things like neurons. Neurons communicate extensively with one another to form memories that neuroscientists study. Neurons eventually die with the progression of Alzheimer’s and related dementias.”
Norris says astrocytes have many branches that wrap around blood vessels in the brain. These branches also wrap around many of the connections between neurons. Because of this arrangement, astrocytes are well-positioned to help deliver energy, nutrients and oxygen from blood to neurons.
This recent publication looks specifically at small cerebral vessel disease. “Cerebral vascular pathology is the second leading cause of dementia behind Alzheimer’s disease, and many people with Alzheimer’s disease also have cerebral vascular pathology,” said Norris. “So it is a really common cause of brain dysfunction, cognitive decline, and memory deficits.”
Norris says this portion of the project aims to understand the mechanisms that couple cerebrovascular pathology to brain dysfunction. He says that approach plays into their interest in reactive astrocytes, as these cells act as a “middleman” between neurons, which demand energy when active, and cerebral vessels, which deliver that energy via the blood.
“Astrocytes become reactive when there is blood vessel damage, and when there’s things like amyloid deposits with Alzheimer’s disease, brain injury, or chronic degeneration,” said Norris.
Through creating these situations with mouse models, researchers found that blood flow in the brain can improve by treatments that prevent or reduce astrocyte reactivity. “And improved blood flow leads to better cognitive function,” said Norris.
This is just one signaling pathway with astrocytes. The plan for this project is to go in and target other signaling pathways to see if they have similar effects.
The study utilized multi-photon microscopy; a type of brain imaging captured in the photo by Sompol for the journal cover. “This imaging gives really good resolution, allowing you to see single capillaries along with the astrocytes that wrap around those capillaries,” said Sompol.
The technology is a key piece to this work. MRI and PET scans are important brain imaging tools, especially for humans. However, they do not allow a focus down to individual cells and blood vessels. “In contrast, multiphoton microscopy allows you to look into the brain of a completely intact, awake animal, and see how individual cell types communicate with each other and communicate with blood vessels,” said Norris.
The P01 award behind this publication (and more work to come) is from the National Institute on Aging (NIA) of the National Institutes of Health (NIH). The award exemplifies team science as it helps support about 35 researchers across six different labs who will be working on four main projects, all with a common theme.
“Neuroscience is a very multidisciplinary field, you really have to approach these problems from many different perspectives,” said Norris.
This is a big reason why the team at Sanders-Brown is situated for work like this which is considered a first of its kind. Reactive astrocytes have been studied for a while now, however Norris said that cell-specific targeting of astrocytes is a relatively new approach. “This is the first study we know of to really look at how reactive astrocytes are affecting blood flow in the brain, which is important because blood flow is reduced in Alzheimer’s disease and other forms of dementia,” said Norris.
The overall venture by the team is made up of four separate projects being led by worldwide leaders in their respective fields. Donna Wilcock, Ph.D., will study astrocyte connections around blood vessels; Pete Nelson, M.D., Ph.D., is leading a project studying a potassium channel acting as a metabolic sensor in astrocytes; and the lab of Olivier Thibault, Ph.D., will examine how astrocytic insulin receptors help to control metabolism, blood flow and calcium homeostasis. Norris is directing a project on astrocytes and the uptake of glutamate, an excitatory neurotransmitter. Sompol along with Yang Jiang, Ph.D., and Yuriko Katsumata, Ph.D., each direct critical service cores supporting the four research projects.
Norris says astrocytes are an appealing cell to study for therapeutic potential as their actual function and contribution to pathology have been taken for granted for decades.
“If we can figure out what reactive astrocytes are doing in terms of pathology, then we can perhaps develop treatments to target those pathways and hopefully alleviate pathology,” he said.
There currently is not a cure for Alzheimer’s disease, and treatment options that have been FDA-approved are limited.
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number P01AG078116. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
More from this series Research Priorities - Neuroscience
Credits
Elizabeth Chapin (Research Communications)
Photo: Arden Barnes (UK Photo)


