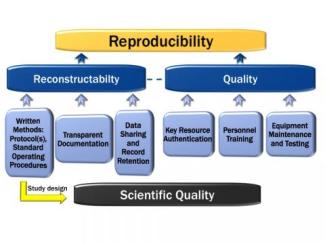
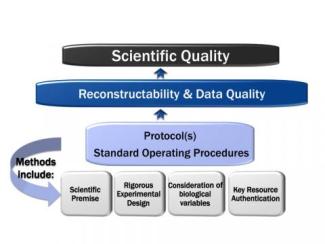
It is the center’s mission to support researchers through the development and support of Good Research Practices (GRPs). GRPs embody study planning, conduct, and reporting principles that share the intent of federal regulations (GLPs, see below).
Good Research Practices (GRPs) support:
- Meeting updated NIH/NSF and journal requirements for conducting and reporting research1-7
- Increased confidence in decisions based on reliable data
- Accessing industry partnerships more readily, and earlier