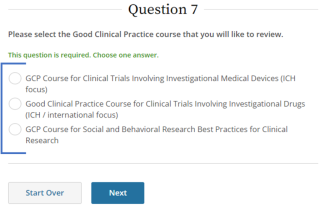
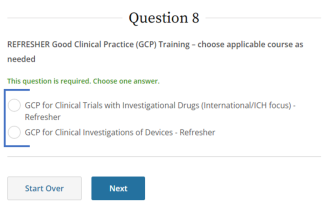
IRB policy requires mandatory training for investigators who are also serving as sponsors, (e.g., holding an Investigational New Drug (IND), Investigational Device Exemption (IDE), or abbreviated IDE), for an FDA-regulated clinical investigation. A Sponsor-Investigator must complete the applicable drug or device Good Clinical Practice training on CITI before final IRB approval is granted. The ReGARDD Training Modules on IND Sponsor-Investigator Responsibilities may be substituted for the CITI GCP course by providing completion documentation to ORI (HSPTrainingSupport@uky.edu).
- If the Sponsor-Investigator has documentation of completion of equivalent training, email course completion documentation to HSPTrainingSupport@uky.edu.
Sponsor-Investigators may also choose to require completion of the training by staff who will be involved in the conduct of the study. This is encouraged for sub-investigators to whom any sponsor responsibilities will be delegated.