The UK curriculum on CITI includes an optional course on Humanitarian Use Devices (HUD). The course is designed for clinicians who seek Institutional Review Board (IRB) approval for the clinical use of a HUD and for clinical researchers who wish to use a HUD in a clinical investigation. Completion of the course may be required at the discretion of the IRB for new HUD users.
FAQs Humanitarian Use Device (HUD) Training
What training is available for clinicians or researchers using Humanitarian Use Devices (HUD)?
How do I access the CITI HUD training module?
The course is available as a stand-alone course and as an optional portion of the Good Clinical Practice (GCP) and Device Development for Sponsor-Investigators GCP courses.
The stand-alone HUD course is available under the IRB menu, Optional Courses.
You may enroll initially from the Courses page after creating your account. Scroll to the bottom of your Courses page then click "Add a Course.
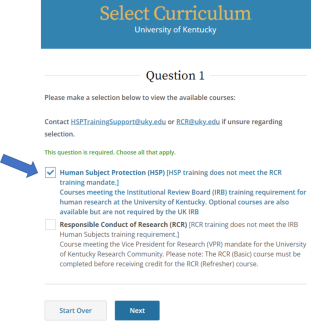
Select Human Subject Protection (HSP), then click Next.
Select Additional Optional Courses of Interest, then click Next.
Select Humanitarian Use Devices (HUD), then click Next.
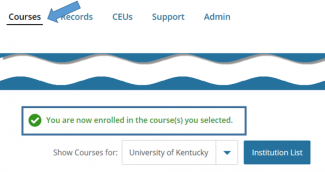
You are directed back to your Courses page where it states you are now enrolled.
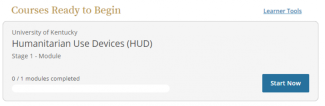
Scroll down to "Courses Ready to Begin" and click Start Now to complete the HUD training.